How much does it cost to breathe local oxygen for a day on Mars?
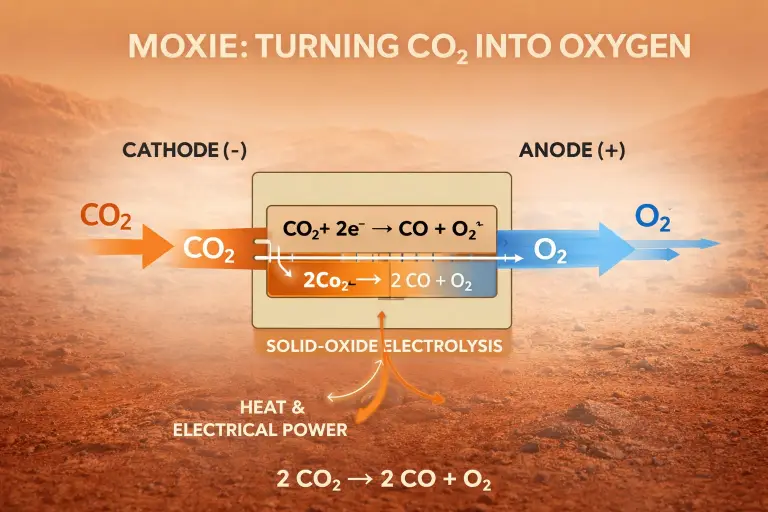
Only proven technology to produce oxygen on Mars is Moxie unit installed on Perseverance Mars Rover since 2021. MOXIE (the Mars Oxygen In-Situ Resource Utilization Experiment) produces oxygen using solid-oxide electrolysis of carbon dioxide. Here’s the chemistry clearly and precisely.
2 CO2 → 2 CO+O2
This reaction splits carbon dioxide, releasing molecular oxygen.
MOXIE operates at ~800 °C using a solid oxide electrolysis cell (SOEC) made with a ceramic electrolyte (yttria-stabilized zirconia).
- CO₂ intake
Mars’ atmosphere (~95% CO₂) is filtered, compressed, and heated.
- Cathode reaction (CO₂ reduction)
At the cathode: CO2+2e− → CO+O2−
- CO₂ gains electrons
- Carbon monoxide is released
- Oxygen ions move through the solid electrolyte
- Oxygen ion transport
- O2− ions migrate through the ceramic electrolyte under an electric field.
- Anode reaction (oxygen formation)
At the anode: 2 O2− → O2+4e−
- Oxygen gas is released
- Electrons are returned to the circuit
Net electrochemical process: 2 CO2 →electric power 2 CO+O2
Key point:
This reaction is not spontaneous. It requires:
- High temperature
- Electrical energy
- Specialized materials
This is why bombs can’t replace MOXIE—you need controlled electrochemistry, not brute force.
In MOXIE:
- CO is vented back into the Martian atmosphere
In future systems:
- CO could be used as:
- Fuel feedstock
- Carbon source for plastics
- Reactant for methane synthesis (Sabatier process)
MOXIE produced up to 12 g of O₂ per hour, enough to show scalability.
Energy required to produce 1 kg of O₂ from CO₂
Theoretical minimum
≈ 4 kWh per kg of O₂
This is the thermodynamic minimum set by electrochemistry.
Practical / real-world value
≈ 15–25 kWh per kg of O₂
This is what you should expect for an actual Mars system like MOXIE or its scaled-up successors.
- Electrochemical minimum
MOXIE uses this reaction:
2 CO2→2 CO+O2
4 electrons per O₂ molecule
- Minimum electrical energy ≈ 463 kJ per mole of O₂
- 1 kg O₂ = 31.25 moles
- Result:
≈4.0 kWh/kg O₂
This assumes:
- Perfect efficiency
- No losses
- No compression or heating costs
Which never happens.
- Real system losses
MOXIE must also:
- Compress Mars’ thin atmosphere
- Heat CO₂ to ~800 °C
- Overcome electrical resistance
- Maintain thermal stability
- Run pumps, valves, control electronics
All of that costs energy.
MOXIE’s demonstrated performance
From published mission data:
- Power: ~300–400 W
- Oxygen output: up to 12 g/hour
- Scaling that up:
MOXIE-equivalent systems ≈ 20 kWh/kg O₂
This matches expectations for early-generation solid oxide electrolysis.
Cost of breathing in Mars
- One astronaut: ~ 0.8 kg O₂/day
- Energy needed: 20 kWh/day per person
- Considering $75 / kWh as energy costs to take solar panels from Earth, breathing Mars oxygen will cost $1,500 per day.
Ultimately by building solar panels & scaling up production in Mars, this will be a fraction of costs.

